Drug application-related document writing
- Clinical Study Reports (CSR): Clinical studies
- Common Technical Document (CTD): Non-clinical and clinical studies
- Queries and responses
- Others (adverse event narratives, protocols, review of ICF etc.)
Article writing
- Review (pathology, treatment, clinical results)
- Clinical trial (RCT, observational research, case reports, retro research, PMS)
- Non-clinical (toxicity, pharmacokinetics, pharmacology)
- Survey (questionnaire, web survey)
We support the entire process of publication which includes searching of submission guideline for target journals, drafting outline, English proofreading, publishing support, and review.
QC
- Check the consistency of various English or Japanese documents
- Check the validity check of various English or Japanese documents
- Check the format
Support for preparation of clinical trial notification (CTN), etc.
- Clinical Trial Notification
- Change Notification
- Discontinuation Notification
- Termination Notification
Project management system
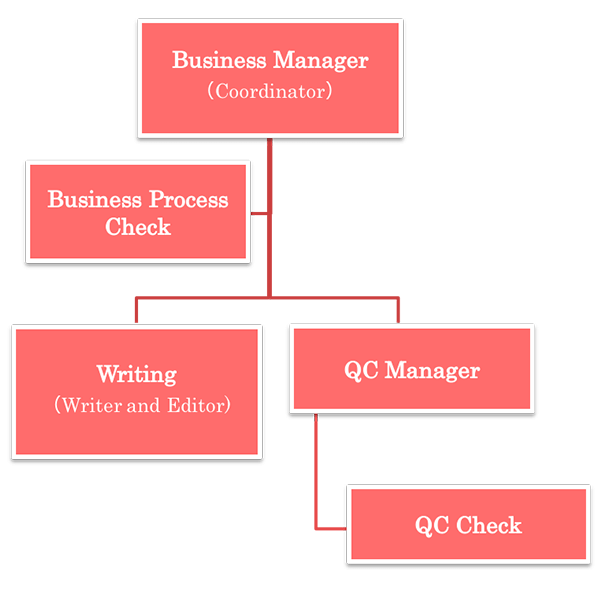
| Function | Roles and Responsibility |
|---|---|
| Business Manager (Coordinator) | Receipt and delivery of related materials and deliverables, point-of-contact for delivery process. Appoint person in charge for each task. Management of work in progress. |
| Writing (Writer and Editor) | Drafting and editing the documents. |
| QC Manager | Check the completeness of quality inspection conducted by QC check (confirm the points checked, any questions or remarks). Other quality inspection related works. |
| QC Check | Quality inspection work of draft documents prepared by the writers. Check the following based on the check list: any content and instructions mismatch, overall consistency. |
| Business Process Check | Check whether the work is performed according to the previously agreed procedures (operation manual, work instructions etc.). |
Major customers
- Pharmaceutical company: more than 30 companies
- CRO:8 companies
- Academia:4 universities