Systematic Reviews and Preparation of Notifications for Foods with Function Claims
Our Academic Expertise Supports Your Evaluation and Documentation with Scientific Evidence
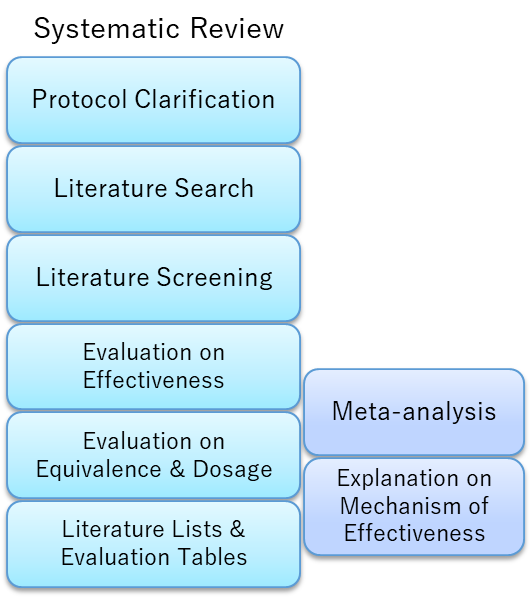
- Writing a systematic review needed for notifications of “Foods with Function Claims” to the Consumer Affairs Agency
- Compliant with the latest notification guidelines and PRISMA 2020
- Literature searches with Web-search queries based on the proposed nutraceutical and the claimed effects
- Primary and secondary screening of literature
- Reviewing literature and evaluating the claimed effect (qualitative review or meta‑analysis)
- Evaluating equivalence, extrapolation, daily intake dosage
- Translation of foreign literature
- Support on writing of other documents (safety evaluation, explanations on mechanisms of effectiveness, etc.) is also provided, please consult with us for details.
- Service Charge:
- Systematic Review (per one claim) minimum 1,500,000 yen (excluding tax)
- Work Period:
Approximately two month from execution of contract to completion of SR (per each claim)
- Forms from (V)-4 to (V)-16 and (V)-18 of the Food Labeling Standards are used
- Creating a new SR or updating existing SRs based on PRISMA 2020
- Service charges and work periods are according to the novelty of the claim or nutraceutical factors, the total number of literature that will be screened, and other factors
- There will be an additional charge and an extended period for Meta-analysis
- SR QC check only is also available
- Inquiries regarding SR organizers will be responded
- Our Clients:
Pharmaceutical Material Manufacturer
Food Material Manufacturer
Health Food Product Manufacturer
Health Food Brand Supplier
Health Food Material or Product Import Agency
Food Contract Research Organization
Contact us by clicking here.
Why Wysiwyg?
- Our company is comprised of human resources majoring in life sciences including Medicine and Pharmaceutics
- We have sophisticated and professional writing skills related to academic articles, applications to be submitted to authorities, and pharmaceutical safety management
- We have extensive experience in summarizing academic articles written in Japanese or foreign languages
- Language is no barrier for us because we deal with foreign articles and information on a daily basis
- We provide many high-quality services